Lab. Life Sci. for Extremophiles
Faculty of Human Sciences, Waseda University
Basic research for developing enzymes with both thermostability and low-temperature activity towards promoting the use of enzymes
Living organisms, including humans, undergo chemical reactions necessary for survival
within their bodies. These reactions occur to create materials for the body and obtain the
energy necessary for life, and they are facilitated by enzymes, which work to make these
reactions more likely to occur. There are already attempts to use these enzymes outside of
cells and apply them to human society, with practical applications such as detergent
additives and food and beverage manufacturing.
Enzyme utilization is already widely used in food and beverage processing. For example,
some infants and adults experience discomfort after consuming milk due to an inability to
effectively degrade lactose, a sugar in milk. To address this issue, lactose-degrading
enzymes are used to selectively degrade lactose in dairy products such as milk, enabling
lactose-intolerant individuals to consume them without difficulty.
Reactions that would take hundreds of years without enzymes can be accelerated to occur
in just one second when using enzymes. Enzymes are also highly efficient, generating only
the desired product with 100% efficiency without producing any wasteful byproducts, unlike
other organic or metal-catalyzed chemical reactions. Enzymes are also highly sensitive and
selective, capable of recognizing even trace amounts of substances and distinguishing between
enantiomers, while remaining inert towards unwanted substances. Finally, enzymes have excellent
energy efficiency, allowing chemical reactions to occur under mild conditions such as room
temperature and pressure. Thus, promoting the use of enzymes can help reduce environmental impact,
solve environmental and energy-related problems, and ultimately achieve carbon neutrality.
However, there are also weaknesses in the use of enzymes. One of them is that many enzymes
found in common (micro)organisms are unstable and quickly inactivated. In living organisms,
enzymes are designed to be rapidly inactivated when not needed, but this becomes a significant
disadvantage when utilizing enzymes outside cells.
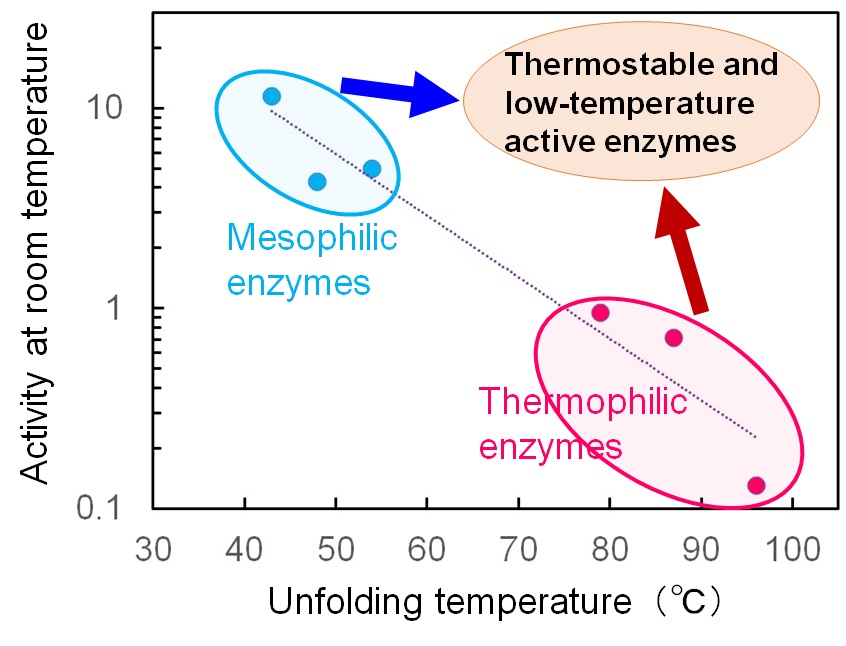
On the other hand, enzymes from thermophilic microorganisms that inhabit high-temperature
environments are stable and active at high temperatures. Although they are more durable and
have a longer half-life than enzymes derived from organisms with lower growth temperatures,
their disadvantage is that they do not work well at room temperature. When using enzymes in
processes where it is difficult to raise the temperature, such as in food and beverage
manufacturing, stable enzymes that are highly active at room temperature are desirable, but
such convenient enzymes are not easily found in nature.
Our laboratory works on the thermostabilization of mesophilic enzymes and the low-temperature
activation of thermophilic enzymes using ancestral reconstruction and evolutionary molecular
engineering methods. In addition, we are reconstructing enzymes that were present in the past
and have since become extinct, aiming to find stable and active enzymes at broad temperatures.